Quality
2021 Sustainability Report
Material Topic: Quality
Quality is a cornerstone of JSR’s values and has been an important component in the company’s continued success. Photoresist and formulated cleans are two central business lines that have benefited from JSR’s commitment to quality. For photoresist, careful quality management is necessary to maintain product consistency for each customer, among highly customized batches. By contrast, formulated cleans is a lean manufacturing and high-yield business in which every unit shipped must chemically match. The rapid growth of formulated cleans during the reporting period has led to a re-evaluation of how to maintain a consistent level of quality while pursuing a notably different business model. As of March 2022, the formulated cleans manufacturing organization has been ISO certified, substantiating successful quality assurance in this growing business.
The Growing Importance of Automation and Data
JSR has significant internal quality data that it is working through to make more accessible and actionable. Within the business units, these data are in a variety of systems that do not necessarily communicate with one another. Our first step has been to break down the quality data silos such that analysis tools can be developed on top of the integrated data sets. The tools under development will synthesize the available data to make quality data easier to enter, manage, learn from, and act upon on a daily basis. For more details about automation and data, please see the Innovation section.
The new formulated cleans manufacturing facility takes advantage of various automation tools to maximize quality. In general, batches of cleans are more sensitive to trace impurities than JSR’s lithography product line. As a result, various automation and software solutions have been implemented to maximize quality performance. For example, a highly accurate metering system automatically dispenses raw materials for each batch of cleans. In the past, if there was a manufacturing issue, a manual data analysis process was performed. It would be a laborious task for an engineer to obtain the correct data and perform the analysis. That process has now been replaced with a cloud-based solution. With this technology in place, data analysis can be performed in just a few minutes. Changes to key parameters can be made and the report can quickly be rerun. This approach has been so successful at the Oregon location that a similar approach is being implemented at the photoresist plant in Sunnyvale.
Quality as a Life Science Differentiator
Given JSR’s legacy of quality management, this has naturally become part of the Life Sciences business. The semiconductor business has some of the most stringent quality standards of any industry, and JSR believes that applying these lessons learned to the biopharmaceutical business represents an opportunity. The goal is to not only meet FDA regulations, but use JSR’s high level of quality control as a differentiator in the life science industry.
Employee Reporting Portal
During the reporting period, we improved our system for gathering input from employees. All employee feedback, including Corrective Action Reports (CARs), Near Misses, and Suggestion Box recommendations are now accessed through a single portal on JSR’s intranet. Previously, these three communication channels were separate, and in different locations, making the process cumbersome and confusing for employees as it was not always clear which one was appropriate for a specific feedback item. The dedicated Employee Reporting Portal now streamlines and guides the process. The portal asks users key questions to ensure that they complete the correct form. This not only reduces reporting errors, it also minimizes friction for employees when submitting feedback to JSR.
SPECIFIC DISCLOSURES, COMPANY SPECIFIC DISCLOSURE: COST OF QUALITY
Maturing Cost of Quality
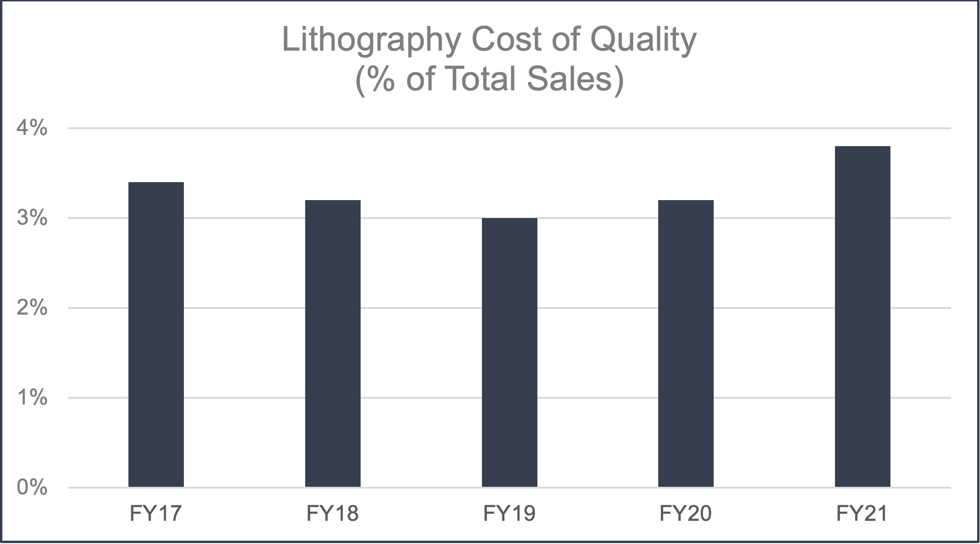
Despite the multi-year global pandemic, JSR has met all of its quality assurance goals during the reporting period and the Cost of Quality (CoQ) for JSR’s semiconductor business has remained around 3%. FY20 and FY21 had a slight increase in CoQ due to investments in an internal Quality Control Laboratory and not because of increased failure costs. JSR believes that CoQ in this range is an optimal balance of cost of the quality program versus quality of product to meet industry expectations.
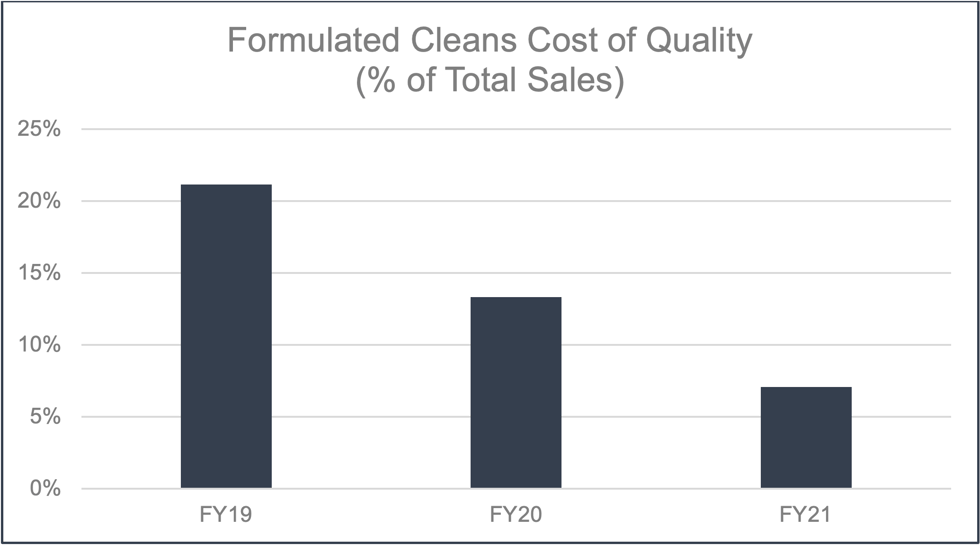
There has been a steep learning curve with the CoQ for formulated cleans. This is the result of formulated cleans being an emerging business with new failure mechanisms and different customer expectations. Going forward it is expected that CoQ will continue to decrease and eventually level off similarly to that of lithography.
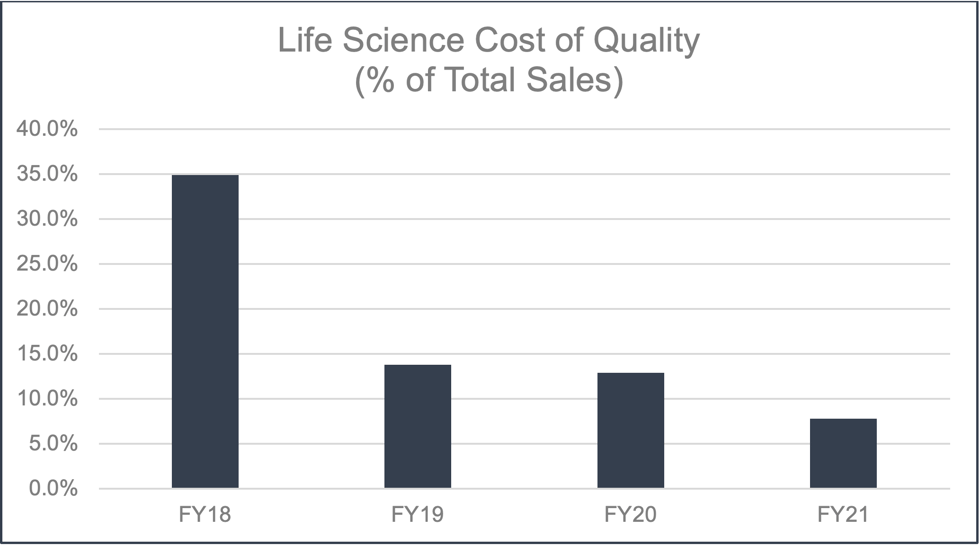
The CoQ for life sciences has demonstrated significant improvement since its introduction in 2019.